Abstract
Introduction: Around 10% of de novo MDS harbor mutations in tumor protein p53 (TP53) gene. Recently, it has been shown that two-thirds of patients with MDS and mutated TP53 gene exhibit multi-hit mutations of TP53, this being associated with poor clinical features and outcome. Taking into consideration that MDS with isolated deletion of chromosome 5 (MDS-del5q) are particularly enriched in TP53 mutations (around 20% exhibit TP53 aberrations), we decided to analyze the TP53 allelic state in patients with MDS-del(5q).
Methods: Patients from seven centers belonging to the Spanish Group of MDS (GESMD) and diagnosed with MDS-del5q according to the WHO 2017 classification were included. Therapy related MDS-del5q, as well as patients on disease modifying therapy prior the genetic analysis were excluded. Clinical characteristics and laboratory data were collected at the moment of diagnosis. Genetic profiling included conventional G-banding analyses, FISH for TP53 deletion, next-generation sequencing (NGS) analysis of a panel of 27 genes recurrently mutated in MDS (ASXL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NPM1, NRAS, PTPN11, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1, ZRSR2), and Sanger sequencing. Variant filtering and categorization were performed according to the Spanish Guidelines (Palomo et al., 2019). Based on TP53 mutations and allelic imbalances in chromosome 17p, patients were classified as follows (Bernard et al, 2020): 1) TP53 wild-type (TP53-wt) when no TP53 mutations were found; 2) TP53 monoallelic (TP53-monoallelic) when only 1 mutation was present; and 3) TP53 multi-hit (TP53-multihit) when multiple mutations or mutation(s) and concomitant deletion or copy number neutral loss of heterozygosity (LOH) were identified. Statistical analysis was performed by R (4.2.0).
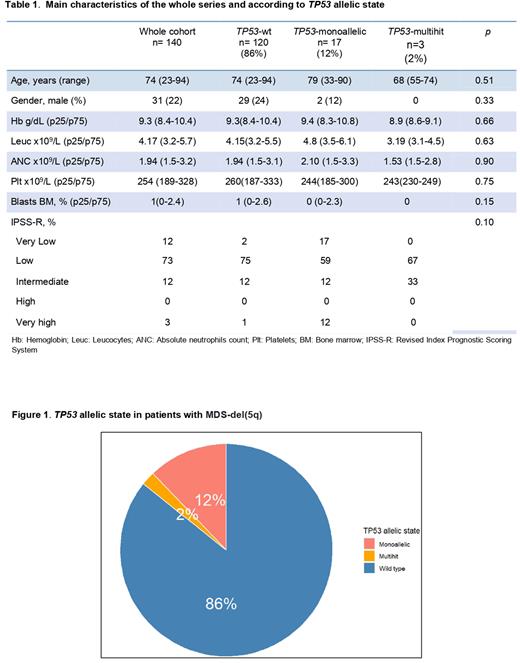
Results: We included 140 MDS-del(5q). Patient's characteristics are detailed in Table 1. Median overall survival (OS) was 96.7 months. Conventional cytogenetic analysis and/or FISH were performed in all cases. NGS was done in 76% (n=106), whereas in the remaining 24% (n=34) analysis of TP53, TET2, CBL and IDH1/2 was performed by Sanger sequencing. Eighty-six per cent (n=120) of patients were TP53-wt, and 14% (n=20) presented TP53 abnormalities: 12% (n=17) TP53-monoallelic and, interestingly, only 2% (n=3) TP53-multihit (Figure 1). In most TP53-monoallelic patients (n=10/17), TP53 mutation was detected as a single genetic event, while the most frequent concomitant mutation among the remaining MDS was DNMT3A (n=3). All TP53-multihit cases harbored two TP53 mutations, but no deletions or LOH in 17p were detected. In 2 out of these 3 patients, no other concomitant mutations were detected. As per the general mutational landscape of the MDS-del(5q) TP53-wt patients, the most frequently affected genes were SF3B1 (15%), DNMT3A (14%), TET2 (13%), ASXL1 (8%), JAK2 (4%), SETBP1 (2%) and CBL (2%). Finally, median OS was 96.7 months for MDS-del(5q) TP53-wt, 62.8 months for MDS-del(5q) TP53-monoallelic, and 56.9 months for MDS-del(5q) TP53-multihit; p= 0.3, with a trend for a worse OS of TP53-monoallelic compared with TP53-wt (HR, 0.5; p=0.106).
Conclusions: This study shows that, as opposite to other MDS subgroups, the presence of multi-hit TP53 status is uncommon in patients with MDS-del5q. Larger series are currently collected to further ascertain the prognostic impact of TP53 aberrations in patients with MDS-del(5q).
Disclosures
Palomo:Janssen: Consultancy. Bosch:Roche: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Karyospharm: Honoraria; Astra Zeneca: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Beigene: Consultancy, Honoraria. Jerez:Gilead: Research Funding; BMS: Honoraria; Novartis: Honoraria. Diez-Campelo:BluePrint: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal